 Members of the Department of Pharmacology investigate signalling mechanisms that communicate the extracellular environment to the cell, and these are subsequently integrated into functional ‘decisions’. Earlier ground breaking studies from Pharmacology group leaders and colleagues established Ca2+ signalling as essential for cell function; this research focus has informed much of current investigation. An overall aim of research in Pharmacology is to identify potential drug targets, which can subsequently be employed to develop strategic therapeutic targets.
Members of the Department of Pharmacology investigate signalling mechanisms that communicate the extracellular environment to the cell, and these are subsequently integrated into functional ‘decisions’. Earlier ground breaking studies from Pharmacology group leaders and colleagues established Ca2+ signalling as essential for cell function; this research focus has informed much of current investigation. An overall aim of research in Pharmacology is to identify potential drug targets, which can subsequently be employed to develop strategic therapeutic targets.
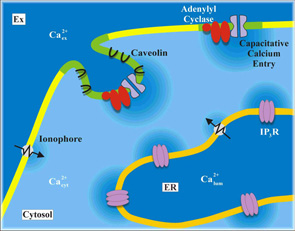
Ca2+ is utilised by many different receptors to control diverse cellular activities, in particular via phosphatidylinositide signals and its regulated release from intracellular stores. This highlights the importance of spatial organization: a Ca2+ signal in one part of a cell can evoke a very different response to a Ca2+ signal than in another part of the same cell. Additionally, the reliability of Ca2+ signals, which involves small numbers of proteins, is poorly understood. To tackle these issues, inositol trisphosphate-evoked Ca2+ signalling is being addressed at levels ranging from atomic structures, through single-molecule functional analyses and organelle dynamics to cellular behaviours (Taylor). Together with Mike Berridge, members of Pharmacology (Irvine) helped to establish inositol 1,4,5-trisphosphate as a second messenger regulating calcium mobilisation. Current focus is on the small family of phosphatidylinositol 5-phosphate 4-kinases, introducing genomic tagging as a technique to give new insights into their cellular targeting and functions.
Many pathways are activated by G protein-coupled receptors (GPCRs). How ligands bind to receptors and activate different pathways is referred to as ‘signal bias’. We look at the ability of ligands for a range of GPCRs to promote either Ca2+ flux, cAMP accumulation, ERK activity or arrestin recruitment (Ladds). Specific interests are directed at how Receptor Activity Modifying Proteins (RAMPs) modulate this sign bias for many family B GPCRs.



