Cyclic AMP
Every aspect in the life of mammalian cells is affected to some extent by cAMP-signaling, from very acute events like regulation of cardiac contraction or neurotransmitter release, to long-term events such as cell growth or development. It is being increasingly recognized that control over so many diverse processes is mediated by a combination of a variety of differently-regulated adenylyl cyclases and phosphodiesterases (the degraders of cAMP) and subcellular targeting of the interacting components. The recognition of this level of organization is now making cAMP signaling a target for very discerning pharmaceutical strategies, which promise far greater specificity than the global strategies of the past. This lab is pitched at the forefront of this compartmentalization arena, developing new tools and perspectives. Techniques in use include a broad range of contemporary molecular and cell biological approaches, as described under the headings below. Four interactive projects are ongoing.
1 - Structure-function studies of adenylyl cyclases
We want to know which structural features render individual adenylyl cyclases susceptible to Ca2+-stimulation or inhibition. We manipulate cyclase cDNAs and express the proteins in either mammalian or insect cells to examine the functional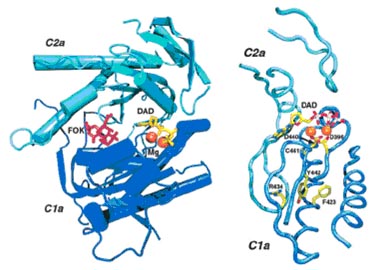 consequences. We are also probing intermolecular associations of the transmembrane-spanning segments of adenylyl cyclases in mediating oligomerization and association with other proteins by the use of CFP- and YFP-tagged constructs, coupled with FRET analysis.
consequences. We are also probing intermolecular associations of the transmembrane-spanning segments of adenylyl cyclases in mediating oligomerization and association with other proteins by the use of CFP- and YFP-tagged constructs, coupled with FRET analysis.
Structure of the catalytic domain of adenylyl cyclase and location of amino acid mutations. Left panel, the forskolin binding site and active site of adenylyl cyclase. The active site occurs at the interface of the C1a and C2a subunits of adenylyl cyclase, which are represented in dark and light blue cartoon format, respectively. The structure is that of the complex of ACVC1a and ACIIC2a (Protein Data Bank code 1CJT) (8). Forskolin, shown in stick representation and in red, binds in the cleft that contains the active site. The ATP analog (DAD, in yellow) and two Mg2+ ions (represented as orange spheres) are bound in the adenylyl cyclase active site in this crystal structure. Right panel, location of the mutated amino acids. The panel shows ribbon representation of the active site and location of the four active site mutations. The two Mg2+ ions are displayed as orange spheres, whereas DAD and the amino acids which are mutated are shown in stick representation. Each residue is coloured by atom (carbon, yellow; nitrogen, blue; oxygen, red; sulphur, pink;). Each of the four mutations occurs proximate to the Mg2+ binding sites. Cys441 and Tyr442 are the two residues just after Asp440, which is of prime importance to Mg2+ binding and adenylyl cyclase activity. The Arg434 and Phe423 are located more distally from the active site. Both residues contact Tyr442, suggesting that mutations of these amino acids might transmit their effects on Mg2+ activation through this residue. Both panels were generated with Visual Molecular Dynamics software (34) and POVRAY (www.povray.org).
2 - Local cAMP and [Ca2+]i signals
We wish to know what interdependent changes occur in these signals in the microenvironment in which they are generated. We use mutated cyclic nucleotide gated ion channels to measure cAMP either by electrophysiological measurements, or fluorimetrically (exploiting the Ca2+-conducting properties of these channels). We also use aequorin-modified adenylyl cyclases to measure Ca2+ in the microdomain of adenylyl cyclase. An extension of these studies is our prediction that cAMP levels may, like [Ca2+]i, oscillate and we are trying to devise methods to determine how targets may respond to cAMP oscillations.
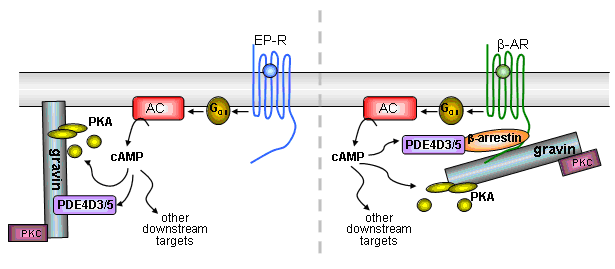
3 - Factors that cause the association of adenylyl cyclases with Ca2+-entry channels
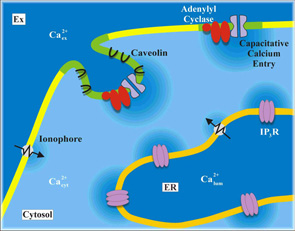
We already know that Ca+--sensitive adenylyl cyclases are exquisitely sensitive to capacitative Ca+--entry in non-excitable cells in the face of much larger elevations in [Ca+-]i arising from either release or ionophore-mediated entry. We are trying to determine by cell biological and molecular biological approaches how this intimate association is maintained.
4 - The cAMP microdomain
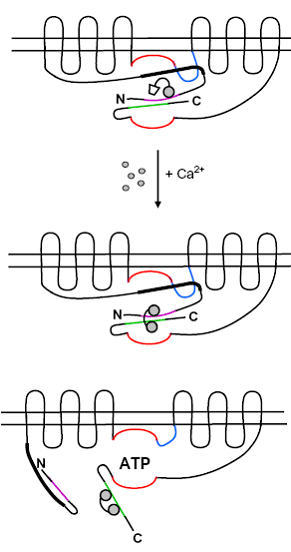 Apart from immediate factors promoting the association of Ca2+-channels with adenylyl cyclases, other proteins and enzymes also contribute to the environment where cAMP originates at the plasma membrane; the role of lipid rafts, calmodulin recruitment, protein kinases, phosphodiesterases and phosphatases, and other factors controlling the macro-environment are also explored.
Apart from immediate factors promoting the association of Ca2+-channels with adenylyl cyclases, other proteins and enzymes also contribute to the environment where cAMP originates at the plasma membrane; the role of lipid rafts, calmodulin recruitment, protein kinases, phosphodiesterases and phosphatases, and other factors controlling the macro-environment are also explored.
Please click on the link if you would like to know more about Cyclic AMP - the rich vein to the Nobel motherlode
