Structure and function of calcium signalling pathways
Our research is concerned with understanding how receptors responding to 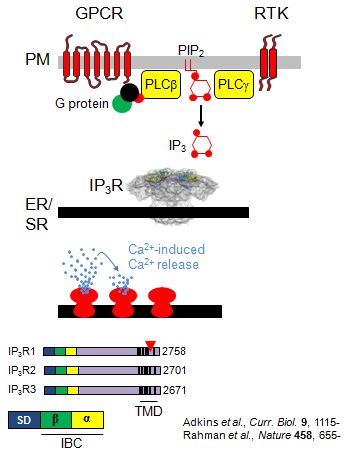 diverse extracellular stimuli evoke release of Ca2+ from intracellular stores and how that then regulates cellular activities. How can the same simple cationic element, Ca2+, be used by so many different receptors to control such diverse cellular activities without wires getting crossed? One recurrent theme is the importance of spatial organization: a Ca2+ signal in one part of a cell can evoke a very different response to a Ca2+ signal in another part of the same cell. A second theme is reliability. How do Ca2+ signals that involve small numbers of proteins in individual cells, and which thereby differ between cells and include considerable randomness, reliably regulate cellular activity? To tackle these issues, we address IP3-evoked Ca2+ signalling at levels that range from atomic structures, through single-molecule functional analyses and organelle dynamics to cellular behaviours. Our work is supported by national and international collaborations with experts in other disciplines that include X-ray crystallography, chemistry and mathematics.
diverse extracellular stimuli evoke release of Ca2+ from intracellular stores and how that then regulates cellular activities. How can the same simple cationic element, Ca2+, be used by so many different receptors to control such diverse cellular activities without wires getting crossed? One recurrent theme is the importance of spatial organization: a Ca2+ signal in one part of a cell can evoke a very different response to a Ca2+ signal in another part of the same cell. A second theme is reliability. How do Ca2+ signals that involve small numbers of proteins in individual cells, and which thereby differ between cells and include considerable randomness, reliably regulate cellular activity? To tackle these issues, we address IP3-evoked Ca2+ signalling at levels that range from atomic structures, through single-molecule functional analyses and organelle dynamics to cellular behaviours. Our work is supported by national and international collaborations with experts in other disciplines that include X-ray crystallography, chemistry and mathematics.
The wasp stings the elephant. How does IP3 gate IP3 receptors?
 Opening of all IP3 receptors is initiated by binding of IP3, a molecule that is some 5000x smaller than the IP3 receptor. How does binding of IP3 cause such a large protein to change its behaviour? IP3 binds to the IP3-binding core located within the N-terminal region of the IP3 receptor. A combination of structure-activity and structural studies demonstrates that all ligands that activate IP3 receptors interact with each side of the clam-like binding site causing it to close. We suggest that this clam closure disrupts interactions between the four IP3 receptor subunits leading to opening of the pore. Ryanodine receptors are the second major family of intracellular Ca2+ channel, and their N-terminal structures and gating mechanism seem remarkably similar to those of IP3 receptors.
Opening of all IP3 receptors is initiated by binding of IP3, a molecule that is some 5000x smaller than the IP3 receptor. How does binding of IP3 cause such a large protein to change its behaviour? IP3 binds to the IP3-binding core located within the N-terminal region of the IP3 receptor. A combination of structure-activity and structural studies demonstrates that all ligands that activate IP3 receptors interact with each side of the clam-like binding site causing it to close. We suggest that this clam closure disrupts interactions between the four IP3 receptor subunits leading to opening of the pore. Ryanodine receptors are the second major family of intracellular Ca2+ channel, and their N-terminal structures and gating mechanism seem remarkably similar to those of IP3 receptors.
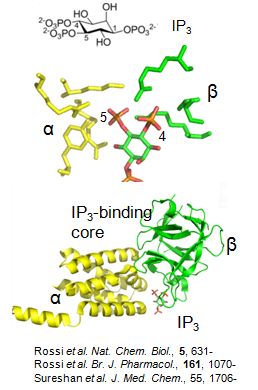 But IP3 alone is not capable of gating IP3 receptors: they must also bind Ca2+. This dual regulation of IP3 receptors by IP3 and Ca2+ is important because it allows IP3 receptors to propagate Ca2+ signals across the cell as regenerative Ca2+ waves. The frequency with which these Ca2+ waves initiate increases as the extracellular stimulus intensity increases. This allows changes in the frequency of the resulting Ca2+ spikes to encode changes in stimulus intensity. But we know very little about the structural basis of Ca2+ binding to IP3 receptors. Another unresolved issue relates to the peculiar pattern of IP3-evoked Ca2+ release from intracellular stores. Stimulation of permeabilized cells with a submaximal concentration of IP3 causes very rapid release of a fraction of the Ca2+ stores, after which release terminates without preventing responses to a subsequent increase in IP3 concentration. This quantal pattern of Ca2+ release is likely to be important in cells responding to physiological stimuli, but its mechanisms are unresolved.
But IP3 alone is not capable of gating IP3 receptors: they must also bind Ca2+. This dual regulation of IP3 receptors by IP3 and Ca2+ is important because it allows IP3 receptors to propagate Ca2+ signals across the cell as regenerative Ca2+ waves. The frequency with which these Ca2+ waves initiate increases as the extracellular stimulus intensity increases. This allows changes in the frequency of the resulting Ca2+ spikes to encode changes in stimulus intensity. But we know very little about the structural basis of Ca2+ binding to IP3 receptors. Another unresolved issue relates to the peculiar pattern of IP3-evoked Ca2+ release from intracellular stores. Stimulation of permeabilized cells with a submaximal concentration of IP3 causes very rapid release of a fraction of the Ca2+ stores, after which release terminates without preventing responses to a subsequent increase in IP3 concentration. This quantal pattern of Ca2+ release is likely to be important in cells responding to physiological stimuli, but its mechanisms are unresolved.
We are presently concerned with answering the following questions:
- What is the structural basis of the interaction between IP3 and Ca2+ binding?
- How are IP3-evoked structural changes within the N-terminal of the IP3 receptor communicated to the distant pore to cause its opening?
- What mechanisms underlie quantal IP3-evoked Ca2+ release.
Whispers and shouts. How are intracellular messengers delivered to IP3 receptors?
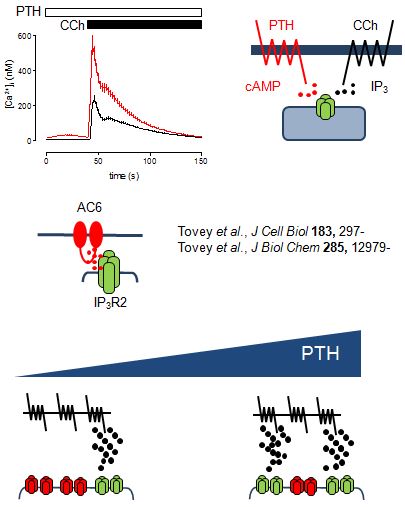
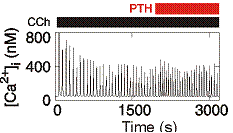
From work examining how parathyroid hormone (PTH) regulates Ca2+ signals, we suggest that cAMP binds directly to IP3 receptors to increase their sensitivity to IP3. In this way, PTH potentiates the Ca2+ signals evoked by receptors that stimulate IP3 formation. Surprisingly, however, even substantial inhibition of cAMP formation has no affect on the ability of PTH to potentiate IP3-evoked Ca2+ signals. This led us to propose that cAMP is delivered directly to IP3 receptors within junctions where the local concentration of cAMP is more than sufficient to maximally sensitize associated IP3 receptors. We further propose that the concentration-dependent effects of PTH come from recruitment of these all-or-nothing hyperactive junctions, rather than from graded increases in activity within individual junctions.
We speculate that similar signalling junctions may allow delivery of IP3 to IP3 receptors. This has important implications. Firstly, it suggests mechanisms by which different receptors may use IP3 to activate different IP3 receptors and thereby evoke different Ca2+ signals. Secondly, receptors in the plasma membrane may either shout or whisper to IP3 receptors. IP3 receptors within signalling junctions will be robustly stimulated by receptor activation, while more distant IP3 receptors will be only weakly stimulated and so perhaps respond only with help from additional messengers like cAMP. Finally, competitive antagonists of IP3 will differentially affect signalling from the plasma membrane to junctional and extra-junctional IP3 receptors.
We are addressing the following questions:
- How is IP3 delivered from different receptors in the plasma membrane to IP3 receptors?
- How are intracellular Ca2+ stores organized to allow different IP3 receptors to release Ca2+ independently?
- How does cAMP regulate IP3 receptors?
Intimate liaisons. How do dynamic proteins and organelles shape Ca2+ signals?
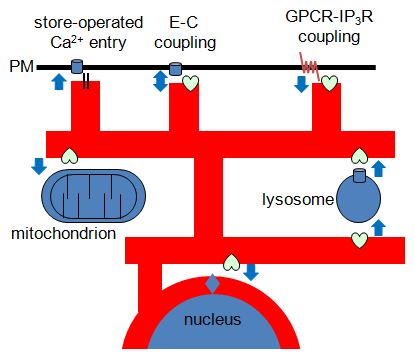
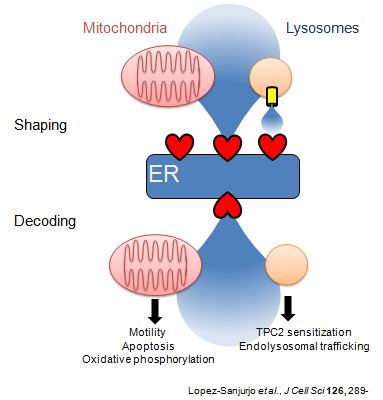
Most IP3 receptors are expressed within membranes of the ER. But the ER is unique amongst intracellular organelles in the extent to which it forms close associations with the plasma membrane and with the membranes of most other intracellular organelles. These interactions have important consequences for both generating and decoding of Ca2+ signals. Furthermore, the ER and the organelles with which it interacts are dynamic, and so too are the proteins, like IP3 receptors, that reside in ER membranes. How are these dynamic proteins and organelles regulated and how do the interactions between them shape cytosolic Ca2+ signals?
 Fluorescence recovery after photobleaching (FRAP) analyses of IP3 receptor mobility and patch-clamp recording of nuclear IP3 receptors suggest that IP3 receptors are mobile within ER membranes and that IP3 causes IP3 receptors to assemble into small clusters. We suggest that this clustering retunes the regulation of IP3 receptors by IP3 and Ca2+ better to allow them to mediate regenerative recruitment of Ca2+ signals. But other evidence has suggested that IP3-evoked Ca2+ signals are initiated by immobile IP3 receptors.
Fluorescence recovery after photobleaching (FRAP) analyses of IP3 receptor mobility and patch-clamp recording of nuclear IP3 receptors suggest that IP3 receptors are mobile within ER membranes and that IP3 causes IP3 receptors to assemble into small clusters. We suggest that this clustering retunes the regulation of IP3 receptors by IP3 and Ca2+ better to allow them to mediate regenerative recruitment of Ca2+ signals. But other evidence has suggested that IP3-evoked Ca2+ signals are initiated by immobile IP3 receptors.
Recent work suggests that mobile lysosomes selectively sequester Ca2+ released by IP3 receptors while ignoring that entering the cell via store-operated Ca2+ entry pathways. Lysosomes that are dynamic but closely associated with ER thereby shape the Ca2+ signals evoked by receptors that stimulate IP3 formation. Neither the means by which lysosomes accumulate Ca2+ nor how they maintain such close contact with ER is understood.
Our work with signalling junctions also suggests that signalling pathways in the plasma membrane come into very close contact with IP3 receptors in the ER. Are these interactions dynamic and regulated?
We are presently addressing the following questions:
- What is the relationship between the distribution of IP3 receptors and
 the Ca2+ signals they evoke?
the Ca2+ signals they evoke? - Does IP3 regulate IP3 receptor distribution?
- How do lysosomes accumulate Ca2+?
- How do dynamic lysosomes maintain their associations with dynamic ER?
- Are signalling junctions between the plasma membrane and ER dynamically regulated?
Getting the message. How do cells decode Ca2+ signals?
How do Ca2+ signals selectively regulate different cellular activities? Ca2+ signals are initiated by random events involving tiny numbers of active IP3 receptors, and individual cells differ in their sensitivities to extracellular stimuli. With so much variable and unpredictable behaviour, how do cells reliable encode extracellular stimuli as Ca2+ signals, and how do they then decode the Ca2+ signals?
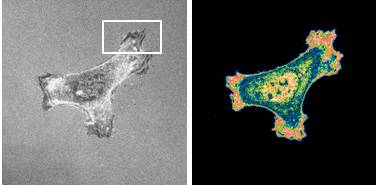 Recent work suggests a role for Ca2+ release via IP3 receptors in controlling cell migration, and we suggest that this may require delivery of IP3 receptors to the leading edge of migrating cells.
Recent work suggests a role for Ca2+ release via IP3 receptors in controlling cell migration, and we suggest that this may require delivery of IP3 receptors to the leading edge of migrating cells.
Analysis of receptor-evoked Ca2+ spikes in a variety of cells suggests that cells respond to increases in stimulus intensity with fold-increases in the frequency of the Ca2+ spikes. This may allow cells reliably to encode changes in stimulus intensity despite considerable randomness and variability.
We are presently addressing the following questions:
- What is the role of IP3 receptor movement in cell migration?
- How is information encoded and then decoded by Ca2+ spikes?

