Submitted by D.P. Juan on Mon, 08/04/2024 - 14:20
Publishing new research is a cornerstone of academic scientific research. Our research groups are regularly publishing their research in a variety of journals.
Below is a list of articles released in March. You can also find a regularly updated RSS publication feed on this page.
Digging deeper into pain: an ethological behavior assay correlating well-being in mice with human pain experience
Pain. 2024 Mar 5. doi: 10.1097/j.pain.0000000000003190. Online ahead of print.
ABSTRACT
The pressing need for safer, more efficacious analgesics is felt worldwide. Preclinical tests in animal models of painful conditions represent one of the earliest checkpoints novel therapeutics must negotiate before consideration for human use. Traditionally, the pain status of laboratory animals has been inferred from evoked nociceptive assays that measure their responses to noxious stimuli. The disconnect between how pain is tested in laboratory animals and how it is experienced by humans may in part explain the shortcomings of current pain medications and highlights a need for refinement. Here, we survey human patients with chronic pain who assert that everyday aspects of life, such as cleaning and leaving the house, are affected by their ongoing level of pain. Accordingly, we test the impact of painful conditions on an ethological behavior of mice, digging. Stable digging behavior was observed over time in naive mice of both sexes. By contrast, deficits in digging were seen after acute knee inflammation. The analgesia conferred by meloxicam and gabapentin was compared in the monosodium iodoacetate knee osteoarthritis model, with meloxicam more effectively ameliorating digging deficits, in line with human patients finding meloxicam more effective. Finally, in a visceral pain model, the decrease in digging behavior correlated with the extent of disease. Ultimately, we make a case for adopting ethological assays, such as digging, in studies of pain in laboratory animals, which we believe to be more representative of the human experience of pain and thus valuable in assessing clinical potential of novel analgesics in animals.
PMID:38452214 | DOI:10.1097/j.pain.0000000000003190
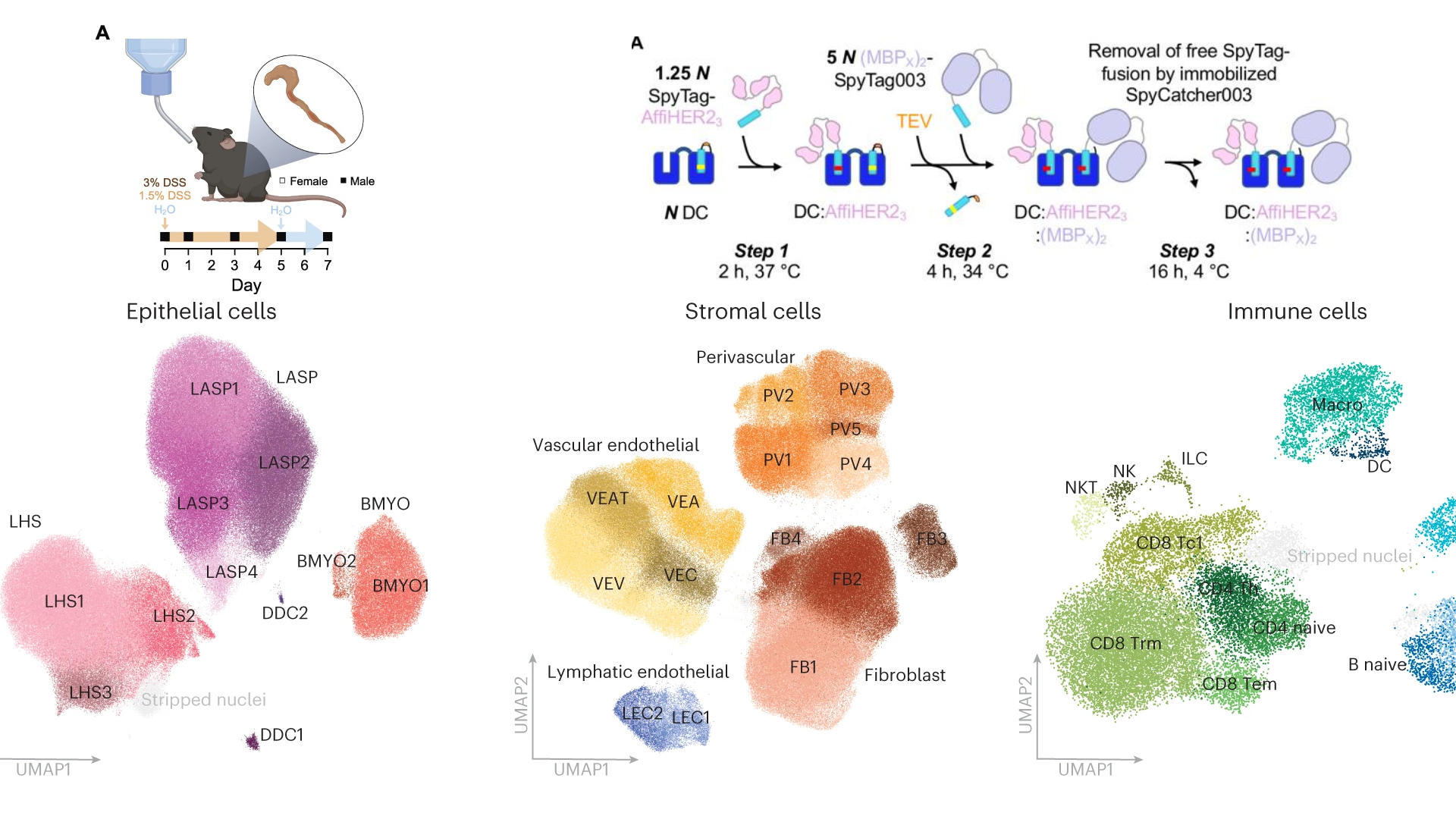
SpyMask enables combinatorial assembly of bispecific binders
Nat Commun. 2024 Mar 16;15(1):2403. doi: 10.1038/s41467-024-46599-9.
ABSTRACT
Bispecific antibodies are a successful and expanding therapeutic class. Standard approaches to generate bispecifics are complicated by the need for disulfide reduction/oxidation or specialized formats. Here we present SpyMask, a modular approach to bispecifics using SpyTag/SpyCatcher spontaneous amidation. Two SpyTag-fused antigen-binding modules can be precisely conjugated onto DoubleCatcher, a tandem SpyCatcher where the second SpyCatcher is protease-activatable. We engineer a panel of structurally-distinct DoubleCatchers, from which binders project in different directions. We establish a generalized methodology for one-pot assembly and purification of bispecifics in 96-well plates. A panel of binders recognizing different HER2 epitopes were coupled to DoubleCatcher, revealing unexpected combinations with anti-proliferative or pro-proliferative activity on HER2-addicted cancer cells. Bispecific activity depended sensitively on both binder orientation and DoubleCatcher scaffold geometry. These findings support the need for straightforward assembly in different formats. SpyMask provides a scalable tool to discover synergy in bispecific activity, through modulating receptor organization and geometry.
PMID:38493197 | DOI:10.1038/s41467-024-46599-9
A single-cell atlas enables mapping of homeostatic cellular shifts in the adult human breast
Nat Genet. 2024 Mar 28. doi: 10.1038/s41588-024-01688-9. Online ahead of print.
ABSTRACT
Here we use single-cell RNA sequencing to compile a human breast cell atlas assembled from 55 donors that had undergone reduction mammoplasties or risk reduction mastectomies. From more than 800,000 cells we identified 41 cell subclusters across the epithelial, immune and stromal compartments. The contribution of these different clusters varied according to the natural history of the tissue. Age, parity and germline mutations, known to modulate the risk of developing breast cancer, affected the homeostatic cellular state of the breast in different ways. We found that immune cells from BRCA1 or BRCA2 carriers had a distinct gene expression signature indicative of potential immune exhaustion, which was validated by immunohistochemistry. This suggests that immune-escape mechanisms could manifest in non-cancerous tissues very early during tumor initiation. This atlas is a rich resource that can be used to inform novel approaches for early detection and prevention of breast cancer.
PMID:38548988 | DOI:10.1038/s41588-024-01688-9
