Submitted by D.P. Juan on Mon, 21/10/2024 - 14:56
Our research groups have been hard at work over the last few months. We've collected all the latest publications for your reading enjoyment here.
You can also find a regularly updated RSS publication feed on this page.
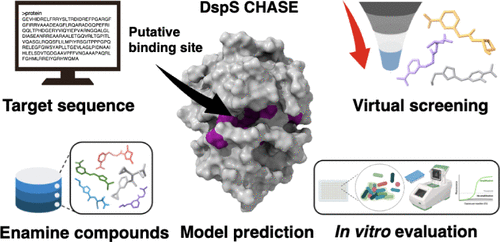
Virtual Screening Uncovers DspS Activators That Disperse Pseudomonas aeruginosa Biofilms
Rahman Group
ACS Infect Dis. 2024 Oct 18. doi: 10.1021/acsinfecdis.4c00549. Online ahead of print.
ABSTRACT
Pseudomonas aeruginosa is the predominant bacterium found in many chronic biofilm infections. Over the past few decades, biofilm-related infections have posed a significant challenge to medical practice due to the increasing emergence of multidrug resistance. Cis-2-decenoic acid (CDA), a small molecule found in P. aeruginosa, has been shown to disperse biofilms formed by various bacteria and to work in synergy with common antibiotics. Despite that, the binding mechanism between CDA and the predicted cyclases/histidine kinases associated sensory extracellular (CHASE) domain of sensor protein DspS remains unknown in the absence of a crystallized protein structure. Moreover, the therapeutic potential of CDA is limited by its susceptibility to oxidative degradation and isomerization. In this work, we propose a structural model for the DspS CHASE domain. The resulting model displays an overall topology reminiscent of the sensor protein PcrK in Xanthomonas campestris. Through molecular dynamics simulations, a stable potential binding site for CDA was further identified. Virtual screening against the predicted site of DspS CHASE using our developed pipeline discovered two promising compounds, compounds 2 and 9, capable of dislodging 7-day P. aeruginosa biofilms at 50 μM without affecting bacterial growth. These compounds also enhanced the effects of ciprofloxacin against P. aeruginosa, reduced the survival of dispersed cells, and increased the expression of matrix-degrading enzyme genes pelA, pslG, and eddA. This study provides insights into CDA recognition by DspS and represents the first large-scale effort to uncover first-in-class DspS activators. At the same time, this work also underscores the effectiveness of a computational-aided drug discovery process in finding new activators, even without a known protein structure.
ER-mitochondria distance is a critical parameter for efficient mitochondrial Ca2+ uptake and oxidative metabolism
Cell Signalling Group
Commun Biol. 2024 Oct 10;7(1):1294. doi: 10.1038/s42003-024-06933-9.
ABSTRACT
IP3 receptor (IP3R)-mediated Ca2+ transfer at the mitochondria-endoplasmic reticulum (ER) contact sites (MERCS) drives mitochondrial Ca2+ uptake and oxidative metabolism and is linked to different pathologies, including Parkinson's disease (PD). The dependence of Ca2+ transfer efficiency on the ER-mitochondria distance remains unexplored. Employing molecular rulers that stabilize ER-mitochondrial distances at 5 nm resolution, and using genetically encoded Ca2+ indicators targeting the ER lumen and the sub-mitochondrial compartments, we now show that a distance of ~20 nm is optimal for Ca2+ transfer and mitochondrial oxidative metabolism due to enrichment of IP3R at MERCS. In human iPSC-derived astrocytes from PD patients, 20 nm MERCS were specifically reduced, which correlated with a reduction of mitochondrial Ca2+ uptake. Stabilization of the ER-mitochondrial interaction at 20 nm, but not at 10 nm, fully rescued mitochondrial Ca2+ uptake in PD astrocytes. Our work determines with precision the optimal distance for Ca2+ flux between ER and mitochondria and suggests a new paradigm for fine control over mitochondrial function.
GPR35 agonists inhibit TRPA1-mediated colonic nociception through suppression of substance P release
Bulmer, Rahman, Smith Group
Pain. 2024 Oct 3. doi: 10.1097/j.pain.0000000000003399. Online ahead of print.
ABSTRACT
The development of nonopioid analgesics for the treatment of abdominal pain is a pressing clinical problem. To address this, we examined the expression of Gi/o-coupled receptors, which typically inhibit nociceptor activation, in colonic sensory neurons. This led to the identification of the orphan receptor GPR35 as a visceral analgesic drug target because of its marked coexpression with transient receptor potential ankyrin 1 (TRPA1), a mediator of noxious mechanotransduction in the bowel. Building on in silico docking simulations, we confirmed that the mast cell stabiliser, cromolyn (CS), and phosphodiesterase inhibitor, zaprinast, are agonists at mouse GPR35, promoting the activation of different Gi/o subunits. Pretreatment with either CS or zaprinast significantly attenuated TRPA1-mediated colonic nociceptor activation and prevented TRPA1-mediated mechanosensitisation. These effects were lost in tissue from GPR35-/- mice and were shown to be mediated by inhibition of TRPA1-evoked substance P (SP) release. This observation highlights the pronociceptive effect of SP and its contribution to TRPA1-mediated colonic nociceptor activation and sensitisation. Consistent with this mechanism of action, we confirmed that TRPA1-mediated colonic contractions evoked by SP release were abolished by CS pretreatment in a GPR35-dependent manner. Our data demonstrate that GPR35 agonists prevent the activation and sensitisation of colonic nociceptors through the inhibition of TRPA1-mediated SP release. These findings highlight the potential of GPR35 agonists to deliver nonopioid analgesia for the treatment of abdominal pain.
Dual regulation of IP3 receptors by IP3 and PIP2 controls the transition from local to global Ca2+ signals
Marti Solano Group, Taylor Group (retired)
Mol Cell. 2024 Sep 28:S1097-2765(24)00742-1. doi: 10.1016/j.molcel.2024.09.009. Online ahead of print.
ABSTRACT
The spatial organization of inositol 1,4,5-trisphosphate (IP3)-evoked Ca2+ signals underlies their versatility. Low stimulus intensities evoke Ca2+ puffs, localized Ca2+ signals arising from a few IP3 receptors (IP3Rs) within a cluster tethered beneath the plasma membrane. More intense stimulation evokes global Ca2+ signals. Ca2+ signals propagate regeneratively as the Ca2+ released stimulates more IP3Rs. How is this potentially explosive mechanism constrained to allow local Ca2+ signaling? We developed methods that allow IP3 produced after G-protein coupled receptor (GPCR) activation to be intercepted and replaced by flash photolysis of a caged analog of IP3. We find that phosphatidylinositol 4,5-bisphosphate (PIP2) primes IP3Rs to respond by partially occupying their IP3-binding sites. As GPCRs stimulate IP3 formation, they also deplete PIP2, relieving the priming stimulus. Loss of PIP2 resets IP3R sensitivity and delays the transition from local to global Ca2+ signals. Dual regulation of IP3Rs by PIP2 and IP3 through GPCRs controls the transition from local to global Ca2+ signals.
