Establishing a structure-function relationship between biomolecular condensates and protein degradation
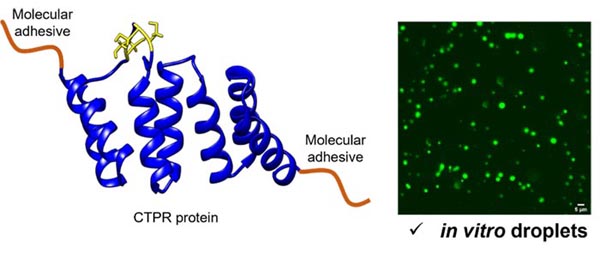
Nature uses liquid-liquid phase separation to rapidly organise biomolecules into condensates that can promote specific reactions and processes. Condensates play an active role in autophagy (the cell’s waste disposal machinery), with their liquid-like nature proving essential for proper autophagic function. We are using protein engineering to create novel synthetic biomolecular condensates and thereby delineate the condensate features that promote protein degradation within the cell. In this way, we will exploit protein homeostasis pathways to drive the removal of disease-causing proteins, such as those associated with neurodegenerative diseases.
To achieve our goals, we will: 1) create novel proteins comprising molecular adhesive peptides to drive liquid-liquid-phase separation (LLPS) and a consensus tetratricopeptide repeat protein (CTPR) to endow the droplets with functional capabilities; 2) characterize LLPS in vitro to define how ligand recruitment modulates the physico-chemical properties of the LLPS-CTPRs; 3) relate the properties of the LLPS-CTPRs to autophagosome formation and subsequent protein degradation; and 4) explore the design of LLPS-CTPRs as a therapeutic strategy to either enhance substrate degradation or disrupt naturally occurring biomolecular condensates.
Linking amyloid structures with cellular dysfunction
Protein misfolding and aggregation in vivo is associated with a wide range of human disorders including Parkinson’s disease, systemic amyloidosis and motor neurone disease. Given the prevalence of these disorders, it is crucial to understand the mechanisms by which disease-associated peptides and proteins behave aberrantly. With this challenge in mind, we aim to define the structural attributes of these elusive species to determine how aggregates form within cells, how they cause cellular dysfunction and how this dysfunction can, in turn, amplify aggregation. By studying this cycle, we will gain a holistic view of the interplay of the processes leading to disease and much-needed strategies for therapeutic intervention.
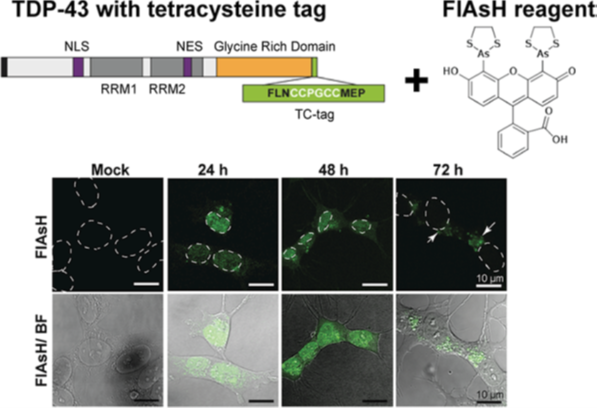
TDP-43 inclusions are characteristic of most forms of amyotrophic lateral sclerosis. Normally a nuclear protein; in the disease-state it translocates to the cytoplasm, forming inclusions that pass from cell-to-cell with detrimental effects. To understand how TDP-43 misfolding and cell-to-cell spread contributes to pathology, it is crucial to examine the structural nature of aberrantly folded protein conformers. We have established a novel mammalian cell model using fluorogenic biarsenical dyes (FlAsH) to monitor the trafficking and aggregation of tetracysteine (TC)-tagged TDP-43 in live cells. The small size of the tag is an advantage over GFP fusions, as it enables live-cell imaging without risk of disrupting protein dynamics. Using this approach we will apply cutting-edge microscopy techniques to follow the spatio-temporal conversion of soluble TDP-43 protein into both amyloid-like aggregates and biomolecular condensates. Resolving these structural alterations within the cell will enable us to determine how exogenous aggregates and co-factors can illicit aberrant TDP-43 behaviour.
Understanding protein homeostasis in relationship to ageing and disease
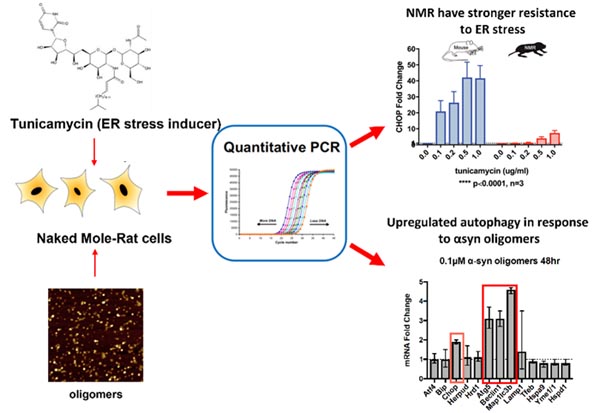
Quality control mechanisms help to maintain a healthy cellular balance; however, the presence of misfolded proteins in age-related diseases is a consequence of the declining efficacy of protein homeostasis (proteostasis). One aspect of the proteostasis network that plays a crucial role in aberrant protein behaviour is ER stress, and in ALS this can induce TDP-43 translocation and subsequent aggregation. Therefore, understanding how proteostasis is impaired by disease is crucial for developing treatments. In collaboration with Dr. Ewan St. John Smith and Prof. Laura Itzhaki, we are investigating proteostasis mechanisms within the naked mole-rat (NMR). This long-living rodent shows negligible age-related impairment of cellular processes, making it a compelling system for studying age-related diseases. We have established transcription-based assays to monitor UPR and autophagy in NMRs, and we will use these tools to investigate how toxic aggregate species elicit changes within these pathways of this unique animal.