Inspired by extraordinary molecular features from the natural world, our research develops new approaches for disease prevention and therapy. By engineering and evolving proteins and cellular systems, our projects range from fundamental analysis of protein interactions through to clinical application.
Immuno-engineering and Global Health
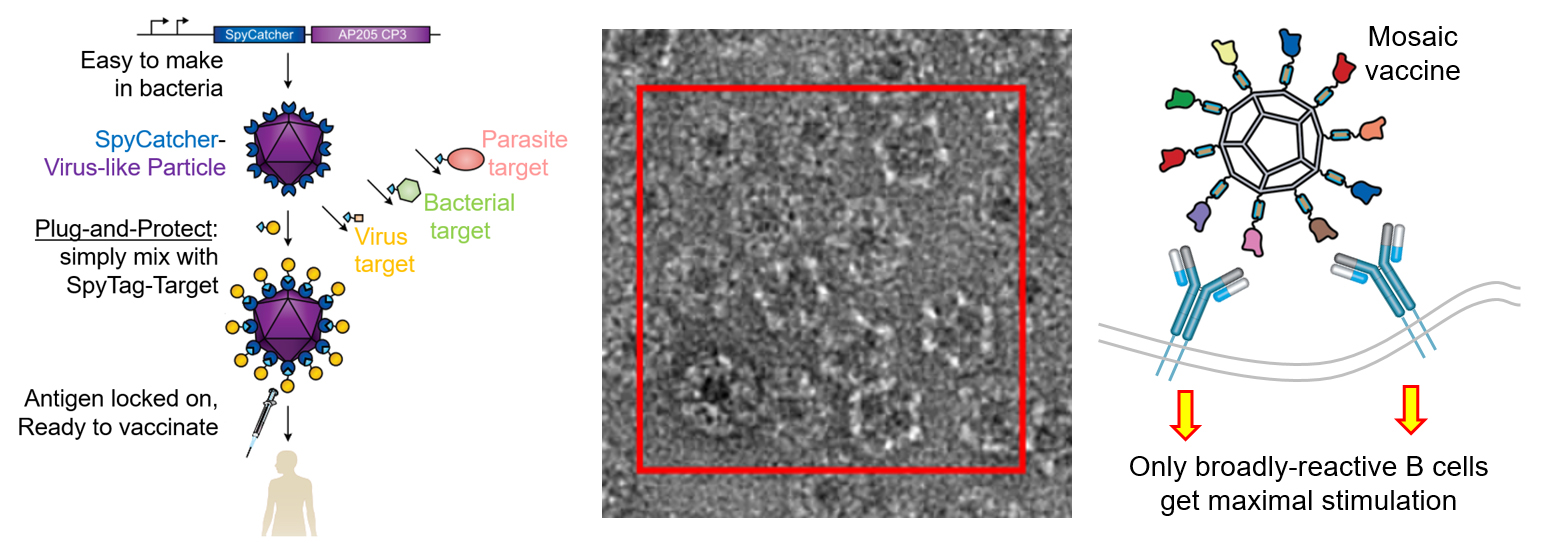
Developing an effective vaccine may be the most effective way to improve human health. We have established an approach to accelerate vaccine development, through our Plug-and-Protect platform. A limiting factor in vaccine generation is the difficulty of turning a promising target protein into the kind of assembly that would give long-lasting disease protection. We showed rapid and efficient decoration of virus-like particles, which elicited a strong immune response even with only a single injection. We have demonstrated potent immunization towards the global health challenge of malaria.
Our platform is now being used by many groups against cancer and various infectious diseases, e.g. HIV, influenza, outbreak pathogens (including SARS-CoV-2), and veterinary diseases. The Plug-and-Protect platform has entered clinical trials for CMV, Covid-19 and malaria, with a pan-sarbecovirus trial in preparation. Our lab’s current focus is to create advances in evolution and computational design for engineering of antigens and protein nanoparticles, as well as establishing principles of immune signalling. Thereby we aim to develop a subsequent generation of vaccine systems, to provide protection against the most urgent disease challenges.
Gastrobodies for targeting in the gut
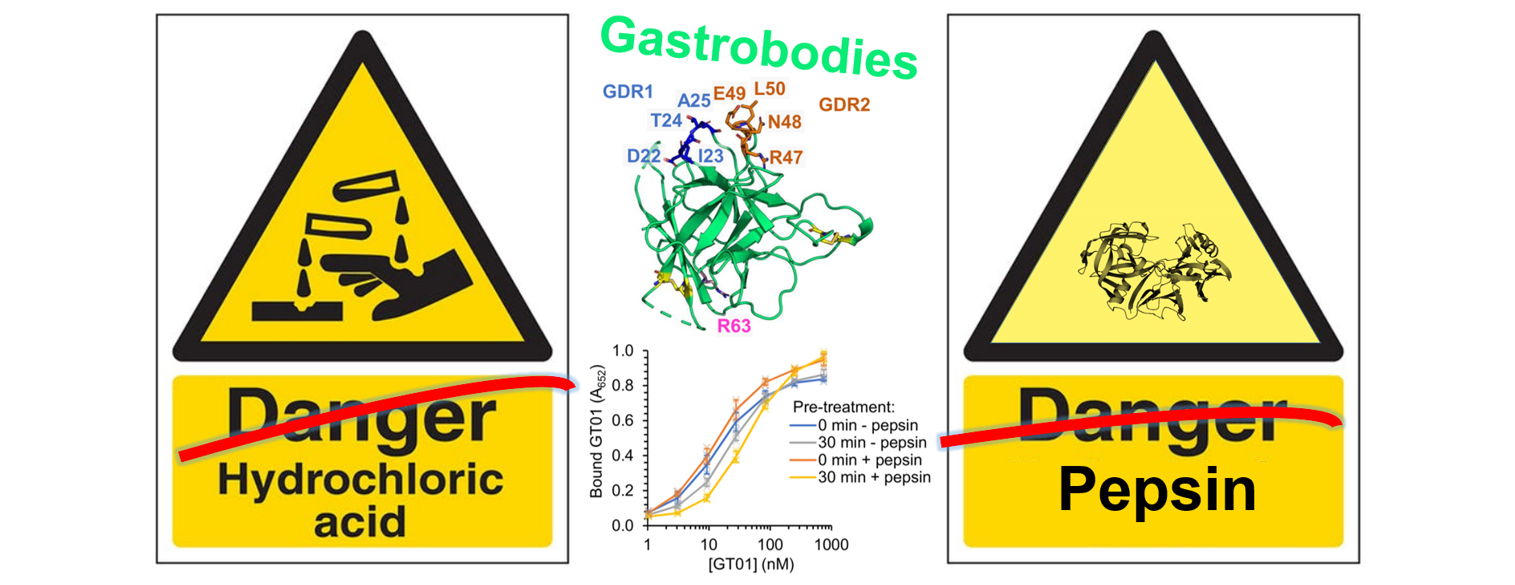
There is an extensive pharmacology of small molecules drugs working successfully in the gut, but the gut is highly effective at degrading proteins. This has prevented the use of antibodies or antibody mimetics for therapeutic targeting in the GI tract. There are a huge range of bacterial/viral infections, cancers and autoimmune diseases where protein-based targeting in the gut could give benefit.
We harnessed a protein from soybean with exceptional resistance to the high hydrochloric acid and pepsin concentrations of the stomach. By computational design and evolution, we established a new antibody mimetic called the gastrobody. Gastrobodies could bind and inhibit a C. difficile toxin important for disease progression. We are currently developing the potential of this new targeting platform towards a range of applications in animal and human health.
Superglues from Bacteria and Antibody Teams
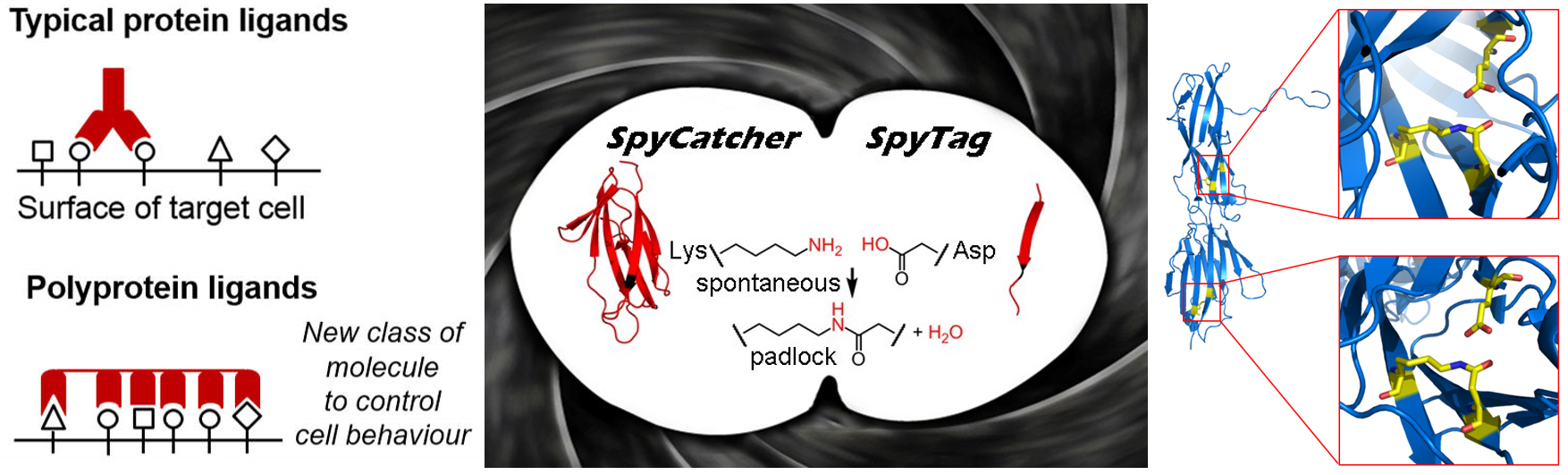
We have harnessed an amazing feature of the hairs (pili) on the pathogenic bacterium Streptococcus pyogenes. This enabled us to form a spontaneous isopeptide bond between genetically-encoded protein and peptide partners. This interaction is unbreakable, including against high forces in biological systems (blood flow, cell migration). Our favourite pair, SpyTag with SpyCatcher, is being applied by hundreds of labs around the world for diverse areas of basic research and biotechnology. We have used computational design and evolution through phage display to create the first genetically-encoded interaction reacting at the diffusion limit and approaching infinite affinity.
Through this principle, we have created a series of protein superglues. We are extending this new class of protein interaction, to create novel possibilities for synthetic biology. One focus is to enhance how antibodies are used and combined: we are building protein teams for combinatorial control of CAR-T cell and cancer cell signalling, towards more precise and potent therapeutics.
Cell Therapy
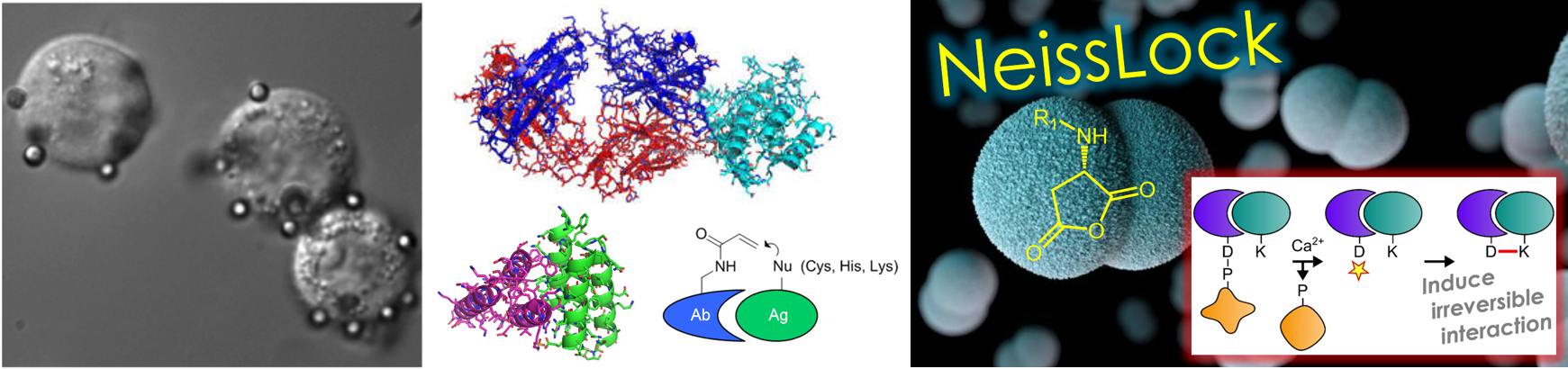
Our studies on the limits of cancer cell capture made clear that even the best antibody interactions are not good enough. We have developed a new class of binding proteins that form covalent bonds to endogenous protein targets. NeissLock was engineered from an adhesion system from Neisseria meningitidis and forms an anhydride in response to calcium, allowing inducible proximity ligation. Protein ligands that never let go of their targets could transform how cells are modified for therapeutic application. Cell therapies can allow pharmacology quite different to small molecule or protein therapeutics. We are advancing NeissLock technology to overcome barriers in cancer immunotherapy and to harness the unique long circulation of red blood cells for sustained therapeutic activity.