G protein coupled receptors (GPCRs) are an extensive family of proteins found across human cells and tissues. Individual family members detect different signals reaching the cell membrane (such as light, taste, neurotransmitters, or hormones) and convey their messages to signalling partners that activate a variety of physiological responses inside our cells. Due to their capacity to regulate cell responses, GPCRs have become the most common drug targets. However, there are still many aspects of GPCR function that remain unresolved, thus hampering our understanding of the key molecular mechanisms that underlie receptor signalling in cell physiology and in therapeutic responses.
In our group, we apply a range of computational biology techniques to mine, integrate and analyse structural, multi-omics, network biology, cell signalling, and pharmacogenomics data. This integrative approach allows us to interrogate GPCR pathways from a systems perspective, revealing general principles governing cell signalling in health and disease that can be experimentally tested by our in house and external collaborators.
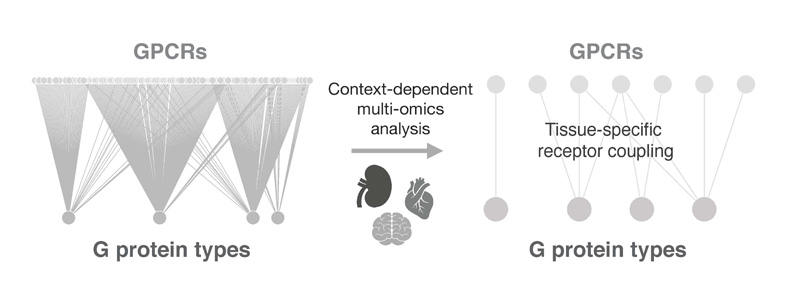
Signalling pathway composition and context-specific responses
Recent work by ourselves and others has revealed the importance of context in GPCR signalling pathway analysis. The exploration of different multi-omics datasets has shown how, while some members of the signalling pathway are ubiquitously expressed, others present selectivity at the cell-type, tissue, organism, or spatiotemporal level. In this way, a more nuanced analysis of pathway composition can uncover how activation of a given receptor can result in an array of signalling responses in different biological systems. Exploiting this knowledge to select specific cellular models that recapitulate pathway composition in our systems of interest will help boosting the translational potential of their signalling readouts.
Structural determinants of signalling diversity
By comprehensively analysing recently available structural data on GPCR pathway components and their modulators we investigate how ligand – receptor recognition and protein – protein interactions have evolved in these signalling systems. This structural understanding also allows us to explore how exogenous ligands like drugs, genomic variation in the general population, or disease-related mutations can modify molecular recognition and receptor dynamics and diversify signalling outputs.
Personalised medicine for GPCR targets
Variability in the wiring of receptor signalling networks in specific physiological and disease contexts can translate into differences in therapeutic response. By incorporating this variability into the study of the therapeutic and side effects of currently available drugs our work aims to suggest new personalised medicine strategies. Importantly, gaining a deeper understanding of the different sources of GPCR signalling variation can guide the selection of new drugs against targets – or even specific receptor-associated pathways – that afford higher selectivity, minimise side-effect risks, and generate more consistent therapeutic responses across the population.