I am interested in developing novel pharmacological approaches to control central nervous system (CNS) activity. My focus to date has been on a family of proteins called g–aminobutyric acid type-A receptors (GABAARs). These ligand-gated ion channels are the principal mediators of inhibitory neurotransmission throughout the central nervous system. They reside at synapses and respond to the release of the neurotransmitter GABA to open an intrinsic chloride channel and pass this electrical response into the post-synaptic neuron. Ordinarily they sculpt neuronal relay by regulating excitation and so moderating CNS activity. In conditions where GABAAR signalling is deficient, neurological disorders arise, such as epilepsy, anxiety and insomnia, all linked to over-excitability. Positive allosteric modulators (PAMs) of GABAARs, such as benzodiazepines, boost GABAAR signalling to treat these conditions. Regulating CNS activity by targeting GABAAR signalling is also linked to other conditions including stroke, autism, pain and schizophrenia.
There are many subtypes of GABAARs and the roles of each in brain function and neurological disorders are far from understood. Superior pharmacological agents are required to discriminate between the subtype roles as part of basic biological research and to develop next-generation therapeutics. My research is aimed at developing strategies to overcome the bottleneck in delivery of selective modulators, and closely relies on biochemical, biotechnological, engineering and electrophysiological approaches. These include: structural biology (X-ray crystallography and cryo-EM) for rational ligand design; dye sensors for ligand screening; raising of protein modulators from immunological libraries; testing of modulators in recombinant and neuronal cell systems; engineering of protein modulators for in vivo delivery across the blood brain barrier into the CNS to enable animal behaviour studies. Progress in these areas will allow scientists to control much more precisely specific types of GABAAR signalling in order to probe basic biology and empower therapeutic design.
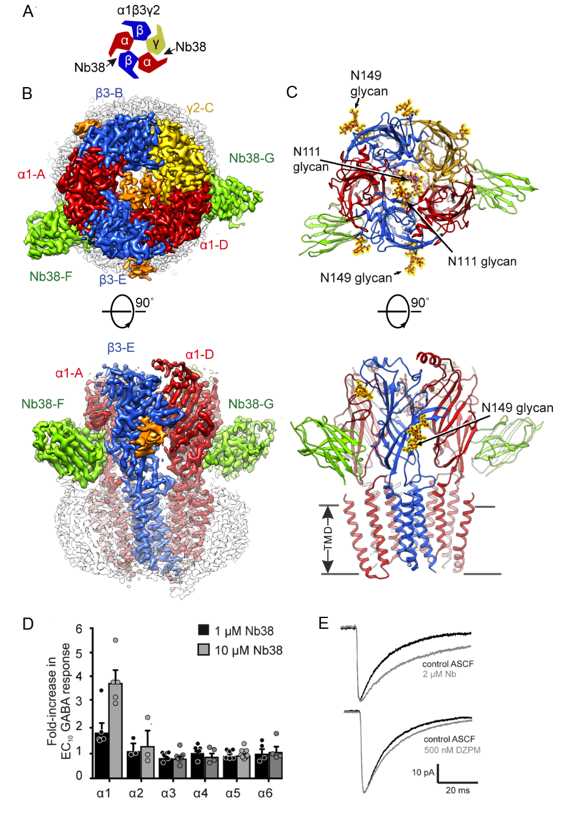
Cryo-EM structure and GABAAR model bound by a nanobody modulator, and its electrophysiological effects. A. Schematic of a GABAAR α1β3γ2 indicating interfaces between subunits that are bound by a nanobody, Nb38. B. The 3.3 Å resolution cryo-EM map (collected on Titan Krios in the Biochemistry facility next door to the Department of Pharmacology), top and side views of GABAAR α1β3γ2. α1 subunits, red, β3 subunits, blue, γ2 subunit, yellow, Nb38, green, detergent belt, white, N-linked glycans, orange. C. α1β3γ2 atomic model. N-linked glycans are in ball-and-stick representation, outlined in yellow. D. Bar chart demonstrating that Nb38 is a selective PAM of α1β3γ2 GABAARs with no potentiation at other subtypes. E. Effect of Nb38 (2 μM) and diazepam (DZPM, 500 nM) relative to control ACSF on average sIPSC waveforms in dentate gyrus granule cells from mouse hippocampus.
